 Hypothyroidism is a condition in which the thyroid gland is underactive and does not produce an adequate amount of the thyroid hormones, T3 and T4. The thyroid hormones are involved in metabolic functions and the generation of body heat.
Hypothyroidism is a condition in which the thyroid gland is underactive and does not produce an adequate amount of the thyroid hormones, T3 and T4. The thyroid hormones are involved in metabolic functions and the generation of body heat.
People suffering from hypothyroidism experience symptoms of fatigue, cold sensitivity, weight gain, bradycardia (slow heart rate), constipation, menstrual irregularities and goitre formation. There are a vast number of causes for hypothyroidism, ranging from dietary insufficiencies, autoimmune diseases and treatment for hyperthyroidism.
Iodine deficiency
Iodine is a dietary element necessary in small amounts to synthesise T3 and T4 from thyroglobulin. The average adult requires 100µg of iodine daily (1). The richest source of iodine in the soil is seawater. Iodine enters the atmosphere in a gaseous form from the sea; thus, coastal areas tend to have richer soil iodine levels than regions further inland (2). Iodine is absorbed from the soil by plants, an important means of ingesting iodine in our diet. Seafood is a rich source of iodine, and in many developed countries food items, such as table salt, are fortified with iodine. Thus, those at risk of developing iodine deficiency tend to live inland or in mountainous regions, have poor access to foods supplemented with iodine and those who exclude seafood from their diet.
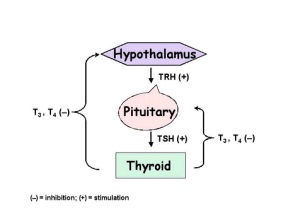
Without iodine, the normal regulatory feedback loop for the thyroid hormones is disrupted. Without enough iodine, insufficient quantities of thyroid hormones are synthesised. Normally, T3 and T4 circulating in the blood act as negative feedback stimuli to the hypothalamus and the anterior pituitary, reducing the quantity of control hormones released by the glands (thyrotropin releasing hormone (TRH) and thyroid stimulating hormone (TSH) respectively). In cases of hypothyroidism, there are insufficient quantities of T3 and T4 circulating to inhibit the release of TRH and TSH. Hence, large quantities of TRH and TSH are released to stimulate the gland to produce thyroid hormones. As there is insufficient iodine available for this process, the thyroid is over-stimulated by TSH, which may lead to goitre formation.
Hashimoto’s thyroiditis
Hashimoto’s thyroiditis is an autoimmune cause of hypothyroidism. In Hashimoto’s thyroiditis, the body produces antibodies to the TSH receptor, thyroglobulin (a precursor to thyroid hormone) or to thyroid peroxidase (an enzyme used to synthesise thyroid hormones) (3). Under normal circumstances, antibodies form our defence to foreign substances; such as bacteria and viruses. In Hashimoto’s thyroiditis, the antibodies are wrongfully directed at the thyroid gland. As a result, the gland becomes inflamed and is unable to synthesise thyroid hormones, inducing a state of hypothyroidism.
There are several risk factors and a number of causative factors which have been linked to Hashimoto’s thyroiditis. Far more women than men suffer from the condition. Several theories are proposed to explain the phenomenon; including the effects of female sex hormones on the immune response and factors related to the X chromosome. Numerous genetic factors have been identified which increase one’s risk for developing Hashimoto’s thyroiditis; some of which have been found due to increased prevalence of Hashimoto’s thyroiditis with other genetic and autoimmune conditions, such as Down’s syndrome, Type 1 diabetes and Turner’s syndrome. The relationship between Hashimoto’s thyroiditis and environmental factors, such as infection and dietary factors, is unclear (4).
After surgery
Thyroidectomy is a surgical procedure in which part or all of the thyroid gland is removed. Thyroidectomy is indicated for some cases of hyperthyroidism, where the thyroid hormones are produced in excess. The aim of surgery for hyperthyroidism is to remove the gland so that thyroid hormones can no longer be synthesised by the gland. As a result, the individual enters a permanent state of hypothyroidism. This can be corrected pharmacologically with a lifelong regime of thyroid hormone replacement, where the patient is given lifelong T4 replacement therapy.
 As a result of treatment for hyperthyroidism
As a result of treatment for hyperthyroidism
Besides surgery, other treatments indicated for treatment of hyperthyroidism are anti-thyroid drugs and treatment with radioactive iodine. As with surgery to correct hyperthyroidism, these non-surgical treatments can in fact render the patient hypothyroid.
Pharmacological treatments for hyperthyroidism largely involve a class of drugs known as thioamides. Common drugs from the thioamide class which are used include propylthiouracil, carbimazole and methimazole. The thioamide drugs have several mechanisms of action to achieve their therapeutic effects. Principally, thioamines inhibit synthesis of thyroid hormones by blocking the enzyme thyroid peroxidase. Thyroid peroxidase is crucial for synthesising T3 and T4 from iodine and thyroglobulin. In addition, the thioamines inhibit the reaction transforming iodide to iodine, and a process called iodotyrosine coupling. Iodotyrosines are formed when iodine is added to the tyrosine (an amino acid) backbones of the thyroglobulin molecule, a step in thyroid hormone synthesis. Iodotyrosines are joined to adjacent iodotyrosines to form either T3 or T4. As the thioamines affect the synthesis of the hormones, rather than their release from the gland, it can take up to 6 weeks for a therapeutic effect to be observed (5).
A second class of drugs which can be used to treat hyperthyroidism are known as anion inhibitors. Examples of anion inhibitors include perchlorate and thiocyanate. These drugs act by preventing the entry of iodide into the cells of the thyroid gland. This is achieved by blocking the sodium-iodide symporter, a transporter in the cell membrane that brings both sodium and iodide into the cell at the same time (5). By blocking the entry of iodide, an essential component of thyroid hormone, synthesis of hormones cannot occur, correcting the problem of hyperthyroidism.
The aim of drug treatment is to reach a euthyroid state for the individual. However, if too high a dose is administered, synthesis of thyroid hormones will be vastly impaired and the patient may actually enter a state of hypothyroidism. If the dose administered is too low, the patient will remain hyperthyroid.
After radioiodine therapy
Radioactive iodine therapy is a non-surgical treatment used to treat hyperthyroidism. The treatment is specific to the thyroid gland because it selectively takes up iodine. This means that the radioactive iodine is only taken up by the thyroid gland and selectively kills the cells of the thyroid. The iodine is not taken up by other tissues, making it a safe and highly specific therapy. However, in 80% of patients, the treatment causes hypothyroidism. The onset of hypothyroidism tends to occur within 12 months of treatment and is an indication that hyperthyroidism has been corrected and will not recur (6). As with thyroidectomy patients, any resulting hypothyroidism is subsequently treated with thyroid hormone replacement therapy.
Congenital hypothyroidism
Congenital hypothyroidism is detected in approximately 1 in 4,000 live births (7). In Australia, every newborn child has a screening test called the Guthrie test, which aims to detect cystic fibrosis, congenital hypothyroidism and metabolic diseases, such as phenylketonuria as early as possible. These diseases may not present obviously in the early years, and the screening test ensures that cases of these diseases can be detected and treated early, minimising the impact on the child. The Guthrie test analyses a sample of blood taken from the heel for a range of clinical markers. For congenital hypothyroidism, an abnormal result is indicated by high levels of TSH and low levels of circulating T3 & T4 (7).
The cause of congenital hypothyroidism can vary. In select cases, congenital hypothyroidism is temporary, resulting from maternal use of anti-thyroid medications or Hashimoto’s thyroiditis. However, most cases of congenital hypothyroidism are permanent. The majority of cases of congenital hypothyroidism are the result of abnormal formation of the thyroid gland during embryonic development. Less common causes include abnormalities of thyroid hormone synthesis and auto-antibody formation against the thyroid gland (8). As per other forms of hypothyroidism, the treatment for the condition is thyroid hormone replacement therapy.
Impaired pituitary or hypothalamic function
The hypothalamus and the pituitary glands control the function of the thyroid gland by release of hormones. Thus, an abnormality of either of these glands can produce hypothyroidism. In instances of hypothyroidism caused by impairments of either the hypothalamus or the pituitary, the resulting condition is termed to be secondary hypothyroidism.
In normal circumstances, when the circulating levels of T3 and T4 fall, the hypothalamus is stimulated to produce and release TRH. TRH travels to the pituitary gland by the hypothalamic-pituitary portal vein and stimulates the production and the release of TSH. TSH then travels to the bloodstream and acts on TSH receptors on the thyroid gland. This is the stimulus for the production and release of thyroid hormones from the thyroid gland. Thus, an abnormality of either the hypothalamus or the pituitary gland resulting in reduced production of TRH or TSH means that there is less stimulation of the thyroid gland, leading to lowered production and release of the thyroid hormones, and thus resulting in hypothyroidism.
If you have questions or concerns about thyroid problems contact your local doctor, who will arrange for you to see a thyroid surgeon.
References
- DeLange, F. (1994). The disorders induced by Iodine deficiency. Thyroid. 4(1): 107-128
- Johnson, C. C., Fordyce, F. M., and Stewart, A. G. (2003). Environmental controls in Iodine Deficiency Disorders Project Summary Report. British Geological survey Commissioned Report CR/03/058N.BGS, Keyworth, Nottingham, UK.
- Bauer D.C., McPhee S.J. (2010). Chapter 20. Thyroid Disease. In S.J. McPhee, G.D. Hammer (Eds), Pathophysiology of Disease, 6e.
- Weetman A.P., Jameson J.L. (2012). Chapter 341. Disorders of the Thyroid Gland. In D.L. Longo, A.S. Fauci, D.L. Kasper, S.L. Hauser, J.L. Jameson, J. Loscalzo (Eds), Harrison’s Principles of Internal Medicine, 18e.
- Dong B.J., Greenspan F.S. (2012). Chapter 38. Thyroid & Antithyroid Drugs. In B.G. Katzung, S.B. Masters, A.J. Trevor (Eds), Basic & Clinical Pharmacology, 12e.
- Cooper D.S., Ladenson P.W. (2011). Chapter 7. The Thyroid Gland. In D.G. Gardner, D. Shoback (Eds), Greenspan’s Basic & Clinical Endocrinology, 9e.
- Bannerman C.G. (2013). Chapter 32. Thyroid & Other Endocrine Disorders during Pregnancy. In A.H. DeCherney, L. Nathan, N. Laufer, A.S. Roman (Eds), CURRENT Diagnosis & Treatment: Obstetrics & Gynecology, 11e.
- Weetman A.P., Jameson J.L. (2012). Chapter 341. Disorders of the Thyroid Gland. In D.L. Longo, A.S. Fauci, D.L. Kasper, S.L. Hauser, J.L. Jameson, J. Loscalzo (Eds), Harrison’s Principles of Internal Medicine, 18e.
