Iodine and Thyroid Hormones
The thyroid gland produces the thyroid hormones T3 (triiodothyronine) and T4 (thyroxine), which help to control the body’s rate of metabolism. Both T3 and T4 have similar actions on the body’s tissues, but T3 is a more potent form of the hormone than T4. The thyroid produces considerably more T4 than T3; however, the body’s cells convert T4 into T3.
Iodine is an important component of both T3 and T4. It is necessary that we consume enough iodine in the diet to produce sufficient T3 and T4 so that the body can maintain the metabolic rate. We need to consume 50mg of iodine (in the form of iodide) per year so that there is enough available to produce sufficient amounts of T3 and T4.
Iodide is absorbed from the intestines into the bloodstream. Iodide from the bloodstream is taken up by the thyroid gland. The thyroid is made up of multiple follicles that are filled with colloid; a substance mainly consisting of fluid and a special type of protein called thyroglobulin.
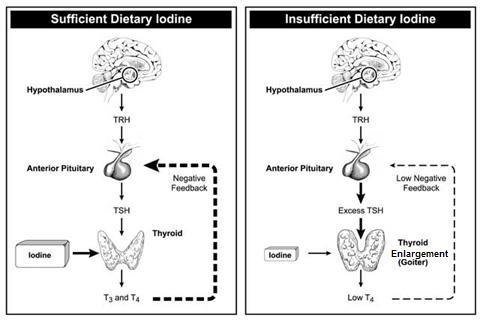
How are the thyroid hormones made?
The first step in the synthesis of thyroid hormones involves transporting iodide from the bloodstream into the follicular cells. There is a special pump on the basal membrane of the cells (faces the outside of the follicle) which line the thyroid follicles and transports iodide into the cell alongside sodium. There is another type of pump which transports sodium out of the cell, called the sodium-potassium pump. The sodium-potassium pump creates a concentration gradient, which favours the transport of sodium into the cell. As iodide is transported with the sodium, the sodium concentration gradient facilitates the movement of iodide into the cell. The follicular cells have a very high concentration of iodide, and the entry of iodine into the cells is promoted by another hormone released from the pituitary gland – thyroid stimulating hormone (TSH).
At the apical membrane of the cells (which faces the centre of the follicle containing the colloid), there is another transporter which moves the iodide out of the cell and into the colloid. This transporter is called pendrin, and it moves iodide into the colloid from the cell in exchange for a chloride ion, which moves from the colloid into the follicular cell. The follicular cells also make and secrete thyroglobulin into the colloid. Thyroglobulin is important because it is the component which binds to the iodide in the colloid to form the thyroid hormones. Thyroglobulin is a protein made from amino acids. The most important amino acid in the thyroglobulin is tyrosine because it is the component which binds with iodine.
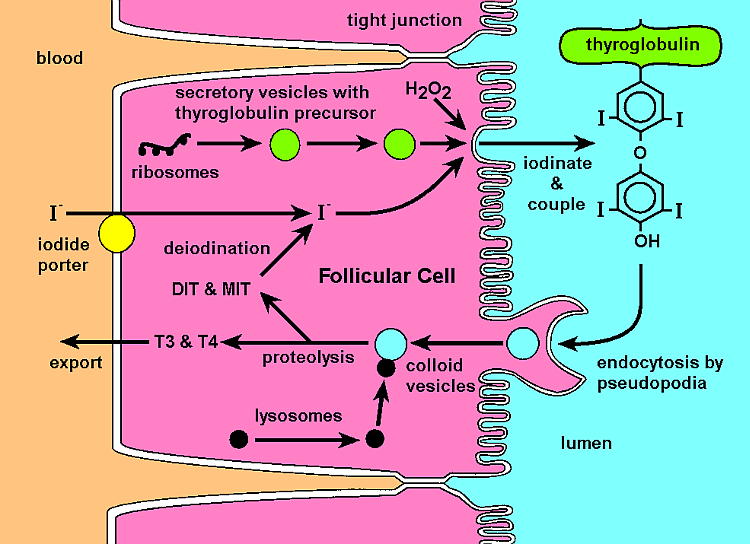
A number of chemical reactions and transformations occur in the formation of the thyroid hormones.
Some important reactions include:
- In the colloid, the iodide undergoes oxidation. Peroxidase is the enzyme which converts iodide to an oxidised form of iodine (I0, I3–). The oxidised forms are able to combine directly with tyrosine.
- Iodine binds to the thyroglobulin in a process that is called organification. Although the oxidised iodine can bind to tyrosine, this is a slow process. Within the thyroid gland, this process is accelerated by the enzyme thyroid peroxidase, which catalyses the reaction between the iodine and the tyrosine so that it occurs within seconds to minutes.
- Once the iodine is bound to the tyrosine residues on the thyroglobulin, a series of iodination reactions occur where more iodine molecules are added to the tyrosine residues.
- The joining of different iodinated tyrosine molecules produces T3 and T4, which are then stored bound to the thyroglobulin in the colloid.
- When T3 and T4 are required by the body, the follicular cell takes up thyroglobulin by the process of pinocytosis, where a droplet of colloid containing thyroglobulin is engulfed by projections of the cell membrane and carried into the cell.
- Inside the cell, the T3 and T4 are cleaved from the thyroglobulin molecule and are secreted by the basal membrane and diffuse into the blood capillaries.
Definition of Iodine Deficiency
Iodine deficiency is not measured in individuals, but rather across populations. It is determined by measuring the concentration of iodine in urine samples of a large proportion of the population in question. In a population, iodine deficiency is defined as a median urinary iodine concentration below 50µg/L [1]. There are varying severities of iodine deficiency, highlighted in Table 1.
| Median Urinary Iodine Concentration (µg/L) | Corresponding Iodine Intake (µg/day) | Iodine Nutrition |
| Less than 20 | Less than 30 | Severe Deficiency |
| 20-49 | 30-74 | Moderate Deficiency |
| 50-99 | 75-149 | Mild Deficiency |
| 100-199 | 150-299 | Optimal |
| 200-299 | 300-449 | More than adequate |
| >299 | >449 | Possible excess |
Table 1: Iodine nutrition status and median population urinary iodine values. Source: American Thyroid Association [1].
The dietary iodine needs for different age groups varies. Thyroid hormones are important for growth and development and the requirement for iodine varies with different stages of life. Pregnant and lactating women, in particular, require more iodine than other population groups. The recommended dietary intake (RDI) is the recommended level of intake for a certain nutrient which is adequate to meet the dietary needs of a healthy individual. For iodine, the RDI for certain population groups are presented in Table 2.
| Age | RDI |
| 1-3 years | 90µg/day |
| 4-8 years | 90µg/day |
| 9-13 years | 120µg/day |
| 14-18 years | 150µg/day |
| Adulthood (19- >70 years) | 150µg/day |
| Pregnancy | 220µg/day |
| Lactation | 270µg/day |
Table 2: RDIs for iodine across different stages of the lifespan [2]
Iodine Deficiency Globally – Endemic vs. Sporadic Goitre
Worldwide, iodine deficiency is a significant public health problem whose ramifications are most serious for children and pregnant women. Iodine deficiency is the leading cause of preventable brain damage in childhood worldwide, and is also a significant contributor to perinatal mortality [3].
Iodine deficiency manifests in different ways across the lifespan as shown below in Table 3.
| Stage of Lifespan | Iodine Deficiency Disorders |
| Foetus |
|
| Neonate |
|
| Childhood and adolescence |
|
| Adulthood |
|
| Elderly |
|
Table 3: Iodine deficiency disorders across different stages of the lifespan. Adapted from: (WHO, 2004) [3].
Intellectual disability and cretinism are the most severe, irreversible ramifications of iodine deficiency disorders; however, goitre is the most noticeable iodine deficiency disorder. There are two subtypes of goitre; sporadic and endemic. Sporadic goitres affect isolated individuals; whereas endemic goitres occur in populations where iodine intake is deficient. Sporadic goitres are more likely to occur in women, those over the age of 40 and people with a family history. Endemic goitre is more of a problem in developing countries because developed countries generally have public health initiatives to increase iodine uptake, such as adding iodine to salt. Endemic goitre also tends to affect inland areas, where the iodine content of the soil is poor.
Goitre
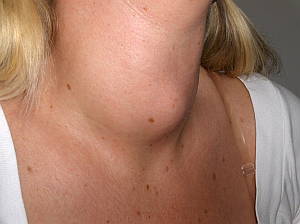 A lack of dietary iodine can cause an enlarged thyroid gland (goitre). Iodine, which is essential for the production of thyroid hormones, is found primarily in seawater and in the soil in coastal areas. In the developing world, people who live inland or at high elevations are often iodine deficient and can develop goitre.
A lack of dietary iodine can cause an enlarged thyroid gland (goitre). Iodine, which is essential for the production of thyroid hormones, is found primarily in seawater and in the soil in coastal areas. In the developing world, people who live inland or at high elevations are often iodine deficient and can develop goitre.
Goitre occurs when the thyroid gland enlarges in an effort to produce thyroid hormone in the absence of sufficient iodine.
The initial iodine deficiency may be made even worse by a diet high in hormone-inhibiting foods, such as cabbage, broccoli and cauliflower. Although a lack of dietary iodine is the main cause of goitre in many parts of the world, this is not often the case in countries where iodine is routinely added to table salt and other foods.
Goitre Belts
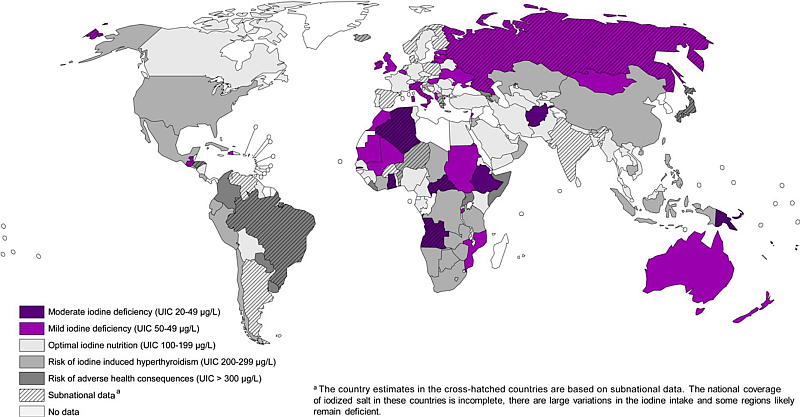
Goitre belts are regions of endemic goitres that are closely correlated with regions of low soil iodine content. There are several goitre belts worldwide which have been well documented. One such goitre belt is in the US and covers the Great Lake, Midwest and the intermountain regions. In the 1900s, up to 70% of children residing in this goitre belt suffered from goitre. However, endemic goitre in the US is no longer an issue. This is due to public health measures, such as the addition of iodine to salt [4]. Although endemic goitre has been largely eliminated in the developed world, significant parts of Asia, Africa and Central and South America are still affected by goitre. A significant goitre belt is found along the Southern slopes of the Himalayas that cover approximately 2,400 kilometres [5]. This goitre belt is situated a considerable distance from the coastline and has limited availability of seafood. Other areas include the Swiss Alps, the Andes Mountains and in central African regions.
Iodine and the Soil
Globally, a major contributing factor to iodine deficiency is a lack of iodine in the soil, especially in inland nations with no coastlines. There are two main mechanisms by which iodine can enter the soil; via precipitation of seawater and by the weathering of rocks which contain iodine. The precipitation of seawater is a more important means of iodine entering the soil than the weathering of rocks. However, just as important as the supply of iodine to the soil is the ability of the soil to retain the iodine – if the soil is richly supplied with iodine but cannot keep hold of it, the supply is futile [6]. A further issue with the iodine status of the soil is the amount of iodine within the soil that is bioavailable – that is, the amount that is free to be taken up by plants. Different types of organic matter in the soil help to retain iodine by fixing it; however, although the soil is rich in iodine, the element cannot be released into plants, and hence the food chain, because it is too tightly bound to the organic matter in the soil [6].
Precipitation
The majority of the world’s iodide is found in the oceans; the iodide evaporates from the ocean and into the atmosphere. The iodide then returns to the soil by the precipitation cycle – the iodide is contained within the rain and enters the soil via rain [7]. Seaweed and phytoplankton are important in releasing iodine into the environment – both are able to produce iodine-containing gases which are released into the atmosphere and further modified by sunlight [6].
Weathering of Rocks
Although less important than the entry of iodine into the soil than precipitation, certain rocks contain iodine which can also enter the soil. When iodine containing rocks become weathered, nearby soils become enriched with iodine [8].
The form of iodine in the soil that is best taken up by plants is iodide. Iodate is another form of iodine, but it is less soluble. The pH of the soil is an important consideration, as acidic (low pH) environments favour iodide, and alkaline (high PH) soil environments favour iodate [6]. Iodine in the soil can exist in solid, liquid or gaseous states; when in the liquid or gaseous state, the iodine is more readily able to enter the plant via its root system, leach into surface waters or be evaporated into the atmosphere. All three of these outcomes make it more likely that the iodine will be available for human consumption [8].
Mountains vs. Valleys
Both mountainous regions and valleys tend to have lower quantities of iodine in the soil, and, therefore, are regions susceptible to iodine deficiency disorders. In Australia, regions of Tasmania and areas of the Great Dividing Range have low quantities of iodine. In older, mountainous regions, the soil iodine content is poor because much of the iodine has been eroded away over time, such as in the Andes Mountains. The iodine content of the soil in valleys is low because valleys are prone to flooding. Floodwaters mobilise and temporarily increase the bioavailability of iodine in the soil, resulting in a long-term depletion of iodine [6, 9].
Inland vs. Coastal
Coastal regions tend to have greater soil concentrations of iodine than inland region. This is related to the entry of iodine into the soil via precipitation. As discussed above, seawater is a rich source of iodine; hence, it is easy to appreciate that areas close to the coast are more likely to have a higher content of iodine in the soil than those further inland, as they receive precipitation with a greater iodine concentration. Analyses of soil, crops and surface water in regions away from the coast have found that all three have a lower concentration of iodine than in coastal regions [6]. Therefore, people living in inland areas are more prone to iodine-deficiency diseases than those living on the coast.
Dietary Sources of Iodine
The major dietary sources of iodine are seafood, iodised salt, eggs and milk. Secondary sources of iodine include meat and cereals [10]. The recommended daily intake (RDI) for iodine will vary based on your age and life stage. In comparison to other nutrients, we only need a very small amount of iodine. The recommendations for adequate dietary iodine intake are taken from the Nutrient reference Values for Australia and New Zealand.
The requirements are measured in micrograms (µg) and are as follows:
- younger children (1 to 8 years) – 90µg
- older children (9 to 13 years, boys and girls) – 120µg
- adolescents (14 to 18 years) – 150µg
- men – 150µg
- women – 150µg
- during pregnancy and breastfeeding – 220µg and 270µg respectively
Seafood
 Seawater is a very abundant source of iodine. Of all foods seaweed, like kelp, is the most famous and reliable source of natural iodine. Marine creatures including oysters, ocean fish and shellfish are all rich in iodine. The level of iodine in freshwater is not as high. Resultantly, freshwater fish are not as rich in iodine as ocean fish [10].
Seawater is a very abundant source of iodine. Of all foods seaweed, like kelp, is the most famous and reliable source of natural iodine. Marine creatures including oysters, ocean fish and shellfish are all rich in iodine. The level of iodine in freshwater is not as high. Resultantly, freshwater fish are not as rich in iodine as ocean fish [10].
Dietitians recommend two to three meals of seafood per week to get the beneficial fish oils. Oysters, sushi containing seaweed, tinned salmon and prawns are excellent sources of iodine. An 80 gram serving of prawns provides 35 micrograms of iodine, or enough to account for just under a quarter of the recommended amount each day. Prawns also provide your body with other essential minerals, primarily protein and calcium.
Milk
In the dairy industry, iodine-based sanitisers (known as iodophores, eg. Betadine) have been used to sanitise equipment involved in processing of milk and other dairy products. This has been a significant iodine source for many Australians. However, iodophores have largely been replaced by antiseptics which do not contain iodine. Hence, iodine deficiency is emerging again as a public health issue in Australia [11]. Historically, Tasmania and areas along the Great Dividing Range have been areas of iodine deficiency. During the 1950s in Tasmania, approximately 50% of children had goitre. The iodine intake to these children was increased through the provision of potassium iodide tablets, iodised salt in bread as well as through iodine intake in milk (which was highly variable between batches) – in fact, Tasmanian people were receiving so much iodine in the end that thyrotoxicosis became a problem in the elderly[11]. Now, iodine use in the dairy industry has exhibited a marked decline. In the 1960s and 1980s, milk iodine levels usually exceeded 500µg/L. In 2001-04, a study of the milk iodine content in Sydney supermarkets revealed that the median value in 2001 was 140µg/L and 195µg/L in 2004 [11].
Salt
 Iodised salt (also spelled iodized salt) is table salt mixed with a minute amount of various salts of the element iodine. The intake of iodised salt in Australia is also declining. Less than 20% of edible salt sold in Australia is iodised, and a lack of legislation surrounding the use of iodised salt in food manufacturing is also contributing to the problem. The problem is further compounded by a lack of knowledge and awareness in the broader Australian community about the health benefits and importance of using iodised salt in cooking. Overall, people are consuming less salt in the diet due to concern about the link between salt intake and cardiovascular disease [11].
Iodised salt (also spelled iodized salt) is table salt mixed with a minute amount of various salts of the element iodine. The intake of iodised salt in Australia is also declining. Less than 20% of edible salt sold in Australia is iodised, and a lack of legislation surrounding the use of iodised salt in food manufacturing is also contributing to the problem. The problem is further compounded by a lack of knowledge and awareness in the broader Australian community about the health benefits and importance of using iodised salt in cooking. Overall, people are consuming less salt in the diet due to concern about the link between salt intake and cardiovascular disease [11].
According to public health experts, iodisation of salt may be the world’s simplest and most cost-effective measure available to improve health, only costing US$0.05 per person per year. At the World Summit for Children in 1990, a goal was set to eliminate iodine deficiency by 2000. At that time, 25% of households consumed iodised salt, a proportion that increased to 66% by 2006.
Bread
In 2009, the fortification of bread with iodised salt at a concentration of 25-65mg iodine per kilogram of salt became mandatory in Australia and New Zealand [12]. Bread was chosen because of its widespread consumption and because it is safe, easy and economically feasible to fortify. However, one issue with the fortification of bread is that it cannot be fortified sufficiently to deliver enough iodine to meet the needs of pregnant and lactating women without causing an excess intake in young children [12]. Initially, a voluntary program was established in Tasmania for the fortification of bread with iodine, which resulted in an improvement in the iodine status of Tasmanian residents. In 2011, a urinary iodine study of Tasmanian school children showed that the concentration of iodine in the urine was significantly higher following mandatory fortification than voluntary fortification [13]. A study analysing pregnant women in Australia also found an increase in the urinary concentration of iodine following mandatory fortification; however, only women also taking iodine supplements had samples reflective of adequate dietary intake during pregnancy [14]. Although fortification is helping to increase iodine intake amongst pregnant women, this population group should also be taking dietary supplements to ensure adequate iodine intake.
Table – Sources of iodine and the amount of iodine they contain
| Food | Iodine Content (µg) per 100g |
| Oysters | 160 |
| Sushi containing seaweed | 92 |
| Canned salmon | 60 |
| Bread baked with iodised salt | 46 |
| Snapper | 40 |
| Cheddar cheese | 23 |
| Eggs | 22 |
| Ice cream | 21 |
| Yoghurt | 16 |
| Milk | 13 |
| Canned tuna | 10 |
| Bread baked with non-iodised salt | 3 |
| Beef, pork & lamb | <1.5 |
| Tap water | 0.5-0.20 |
| Apples, oranges, grapes and bananas | <0.5 |
Table 4: Iodine content of various food items. Source: Nutrition Australia[15]
Summary
Worldwide, iodine deficiency is the leading cause of thyroid disease, as well as intellectual disability. In Australia, iodine deficiency has re-emerged in recent years, despite previous success in increasing dietary intake of iodine through use of iodine-based sanitisers in the dairy industry and addition of iodine to salt. However, changes in practice in the dairy industry surrounding the use of sanitisers and reduced consumption of salt has contributed to the re-emergence of iodine deficiency in Australia. The mandatory fortification of bread with iodine in 2009 has been successful, improving population iodine consumption.
If you have questions or concerns about thyroid health make an appointment to see our thyroid surgeon.
References
[1] American Thyroid Association. (2012). Iodine Deficiency. thyroid.org/iodine-deficiency
[2] National Health and Medical Research Council. (2006). Nutrient Reference Values for Australia and New Zealand including Recommended Dietary Intakes. nrv.gov.au
[3] World Health Organisation. (2004). Iodine status worldwide: WHO Global database on iodine deficiency. whqlibdoc.who.int
[4] Pearce, E. N. (2007). National Trends in Iodine Nutrition: Is Everyone Getting Enough? Thyroid, 17(9). 823-827.
[5] Sooch, S. S. & Ramalingaswami, V. (1965). Preliminary report of an experiment in the Kangra valley for the prevention of Himalayan endemic goitre with iodized salt. Bulletin of the World Health Organisation 32(3): 299-315.
[6] Johnson CC, Fordyce FM, Stewart AG. Environmental controls in Iodine Deficiency Disorders Project Summary Report. 2003; British Geological survey Commissioned Report CR/03/058N.BGS, Keyworth, Nottingham, UK
[7] Yao Y, Pei F, Kang P. Selenium, iodide, and the relation with Kashin-Beck disease. Nutrition 2011; 27(11):1095-1100.
[8] Ashworth DJ. (2009). Transfers of iodine in the soil-plant-air system: Solid-liquid partitioning, migration, plant uptake and volatilization. In VR Preedy, GN Burrow & R Watson (Eds.) Comprehensive Handbook of Iodine: Nutritional, Biochemical, Pathological and Therapeutic Aspects. Elsevier:London
[9] Caballero, B. (2009). Guide to Nutritional Supplements. Elsevier, Oxford.
[10] Thomson, CD. (2004). Selenium and iodine intakes and status in New Zealand and Australia. British Journal of Nutrition, 91(5):661-672
[11] Eastman CJ, Li M. (2009). The current state of iodine deficiency disorders in Australia and the Pacific states. In VR Preedy, GN Burrow & R Wastson (Eds) Comprehensive Handbook of Iodine: Nutritional, Biochemical, Pathological and Therapeutic Aspects. Elsevier: London
[12] Thoma C, Seal J, Mackerras D, & Hunt A. (2011). Iodine fortification of bread: Experiences from Australia and New Zealand. In VR Preedy, RR Watson & VB Patel (Eds). Flour and breads and their fortification in health and disease prevention. Elsevier: Amsterdam
[13] DePaoli KM, Seal JA, Burgess JR, Taylor R. (2013). Improved iodine status in Tasmanian schoolchildren after fortification of bread: a recipe for national success. Medical Journal of Australia, 198(9):492-494.
[14] Charlton KE, Yeatman H, Brock E, Lucas C, Gemming L, Goodfellow A & Ma G. (2013). Improvement in iodine status of pregnant Australian women 3 years after introduction of a mandatory fortification programme. Preventive Medicine, 57(1):26-30.
[15] Nutrition Australia. (2010). Iodine Facts. nutritionaustralia.org